Ag+ is a fascinating topic in chemistry that delves into the electron configuration of silver when it loses one electron to form a positively charged ion. This subject plays a crucial role in understanding the behavior of transition metals and their chemical properties. In this article, we will explore the electron configuration of Ag+ comprehensively, ensuring you have a clear and detailed understanding of this topic.
Transition metals, such as silver (Ag), exhibit unique properties due to their electron configurations. When silver forms the Ag+ ion, it undergoes a change in its electron arrangement, which significantly impacts its reactivity and bonding capabilities. Understanding these changes is essential for anyone studying chemistry, especially those interested in inorganic chemistry and material science.
Our focus will be on providing you with an in-depth analysis of the electron configuration of Ag+. By the end of this article, you will have a solid grasp of the principles behind electron configurations, the significance of Ag+ in chemical reactions, and how this knowledge applies to real-world applications. Let's dive into the world of atomic structure and explore the intricacies of Ag+.
Read also:Alien Encounter In Ruwa Zimbabwe Africa Unveiling The Mysteries
Table of Contents
- Introduction to Electron Configuration
- Properties of Silver
- Electron Configuration of Neutral Silver
- Formation of Ag+ Ion
- Detailed Electron Configuration of Ag+
- Sub-levels and Orbitals
- Importance in Chemistry
- Real-World Applications
- Common Misconceptions
- Conclusion and Next Steps
Introduction to Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes how electrons are distributed among the various orbitals of an atom. It follows specific rules, such as the Aufbau principle, Hund's rule, and the Pauli exclusion principle. These rules ensure that electrons occupy orbitals in a way that minimizes the energy of the atom.
For transition metals like silver, electron configurations can become more complex due to the presence of d orbitals. Understanding these configurations is crucial for predicting the chemical behavior of these elements. The electron configuration of Ag+, in particular, highlights the stability achieved when silver loses one electron from its outermost shell.
Rules Governing Electron Configuration
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level.
- Hund's Rule: Electrons occupy degenerate orbitals with parallel spins before pairing up.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers.
Properties of Silver
Silver (Ag) is a transition metal with the atomic number 47. It is known for its high electrical and thermal conductivity, making it valuable in various industrial applications. Silver also exhibits excellent reflectivity, which is why it is used in mirrors and other reflective surfaces.
In its neutral state, silver has a stable electron configuration. However, when it forms the Ag+ ion, its properties change significantly. This transformation is due to the loss of one electron, which affects its reactivity and bonding capabilities.
Key Properties of Silver
- Atomic Number: 47
- Atomic Mass: 107.87 u
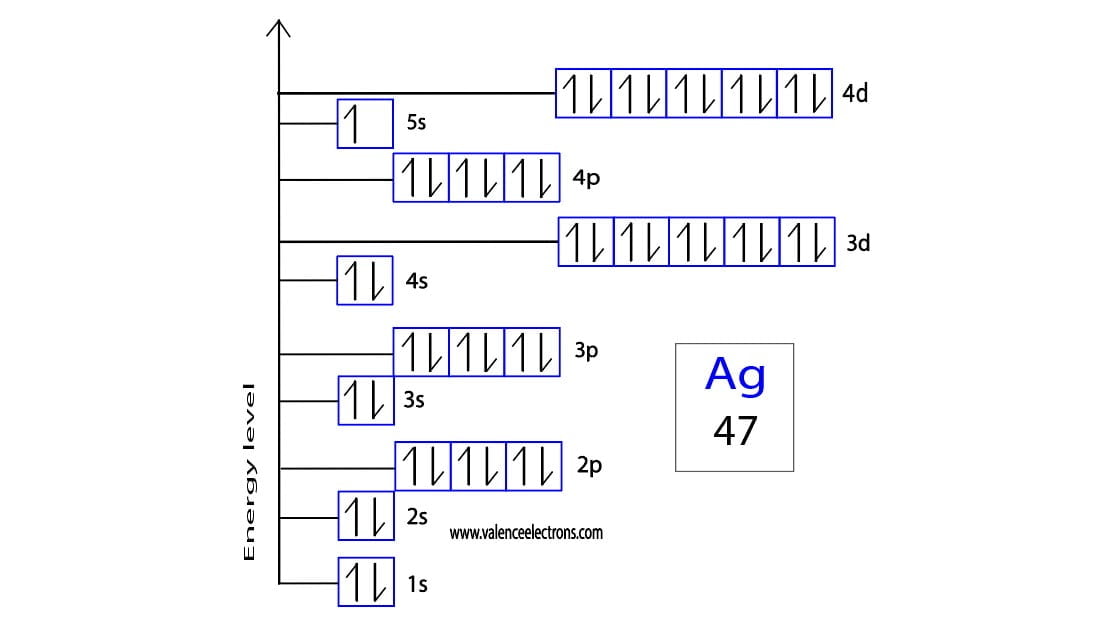
- Electron Configuration: [Kr] 4d¹⁰ 5s¹
Electron Configuration of Neutral Silver
Neutral silver has an electron configuration of [Kr] 4d¹⁰ 5s¹. This configuration indicates that silver has a full 4d orbital and a single electron in its 5s orbital. The presence of a half-filled or fully filled d orbital contributes to the stability of silver in its neutral state.
When silver forms the Ag+ ion, it loses the single electron from its 5s orbital, resulting in a more stable configuration. This loss of an electron is a common occurrence in transition metals, as it allows them to achieve a more stable electron arrangement.
Read also:Body Factory New York Your Ultimate Guide To Wellness And Fitness
Why Does Silver Lose Its 5s Electron?
- The 5s orbital is higher in energy compared to the 4d orbital.
- Removing the 5s electron results in a fully filled 4d orbital, which is more stable.
Formation of Ag+ Ion
The formation of the Ag+ ion involves the removal of one electron from the neutral silver atom. This process occurs during chemical reactions where silver donates an electron to achieve a more stable configuration. The resulting Ag+ ion has an electron configuration of [Kr] 4d¹⁰, which is identical to the noble gas krypton.
This stability is due to the fully filled d orbital, which provides a lower energy state for the ion. The formation of Ag+ is a common occurrence in chemical reactions involving silver, such as the formation of silver nitrate (AgNO₃).
Chemical Reactions Involving Ag+
- Ag → Ag+ + e⁻
- AgNO₃ → Ag+ + NO₃⁻
Detailed Electron Configuration of Ag+
The electron configuration of Ag+ is [Kr] 4d¹⁰. This configuration represents a fully filled d orbital, which contributes to the stability of the ion. The loss of the 5s electron results in a more stable arrangement, as the 4d orbital is lower in energy compared to the 5s orbital.
This stability is crucial for understanding the chemical behavior of Ag+. The fully filled d orbital makes Ag+ less reactive compared to neutral silver, as it has achieved a noble gas configuration. This configuration also affects the bonding capabilities of Ag+, making it an important ion in various chemical reactions.
Comparison with Neutral Silver
- Neutral Silver: [Kr] 4d¹⁰ 5s¹
- Ag+: [Kr] 4d¹⁰
Sub-levels and Orbitals
Understanding the sub-levels and orbitals of silver is essential for grasping its electron configuration. Silver has a complex arrangement of electrons, with the 4d and 5s orbitals playing a significant role in its chemical behavior.
The 4d orbital is fully filled in both neutral silver and Ag+, while the 5s orbital loses its electron in the formation of Ag+. This loss results in a more stable configuration, as the fully filled d orbital provides a lower energy state for the ion.
Key Features of Silver's Orbitals
- 4d Orbital: Fully filled in both neutral silver and Ag+.
- 5s Orbital: Contains one electron in neutral silver, empty in Ag+.
Importance in Chemistry
The electron configuration of Ag+ is of great importance in chemistry, as it influences the reactivity and bonding capabilities of silver. Understanding this configuration helps chemists predict the behavior of silver in various chemical reactions, as well as its role in forming compounds.
Ag+ is commonly found in compounds such as silver nitrate (AgNO₃), which is used in photography, medicine, and other industrial applications. The stability of the Ag+ ion makes it a valuable component in these compounds, contributing to their effectiveness and reliability.
Applications of Ag+ in Chemistry
- Silver Nitrate (AgNO₃): Used in photography and medicine.
- Silver Chloride (AgCl): Used in photographic film and paper.
Real-World Applications
The electron configuration of Ag+ has numerous real-world applications, ranging from industrial processes to medical treatments. Silver compounds, such as silver nitrate and silver chloride, are widely used in various fields due to their unique properties.
In addition to its industrial applications, silver is also used in medical treatments due to its antimicrobial properties. Silver ions, including Ag+, are effective in killing bacteria and preventing infections, making them valuable in wound dressings and other medical products.
Examples of Silver's Applications
- Photography: Silver nitrate is used in photographic film and paper.
- Medicine: Silver ions are used in wound dressings and antimicrobial products.
Common Misconceptions
There are several misconceptions surrounding the electron configuration of Ag+. One common misconception is that silver loses an electron from its 4d orbital instead of its 5s orbital. This misunderstanding arises from the assumption that the 4d orbital is higher in energy, which is incorrect.
Another misconception is that the electron configuration of Ag+ is less stable than that of neutral silver. In reality, the fully filled d orbital in Ag+ provides a more stable configuration, making the ion less reactive and more stable than neutral silver.
Clarifying Misconceptions
- Silver loses an electron from its 5s orbital, not its 4d orbital.
- The electron configuration of Ag+ is more stable than that of neutral silver.
Conclusion and Next Steps
In conclusion, the electron configuration of Ag+ is a crucial topic in chemistry that highlights the stability achieved when silver loses one electron to form a positively charged ion. Understanding this configuration is essential for predicting the chemical behavior of silver and its role in various applications.
We encourage you to explore further by reading related articles and experimenting with chemical reactions involving silver. Your feedback and questions are always welcome, so feel free to leave a comment or share this article with others who may find it useful. Together, let's continue to deepen our understanding of the fascinating world of chemistry!